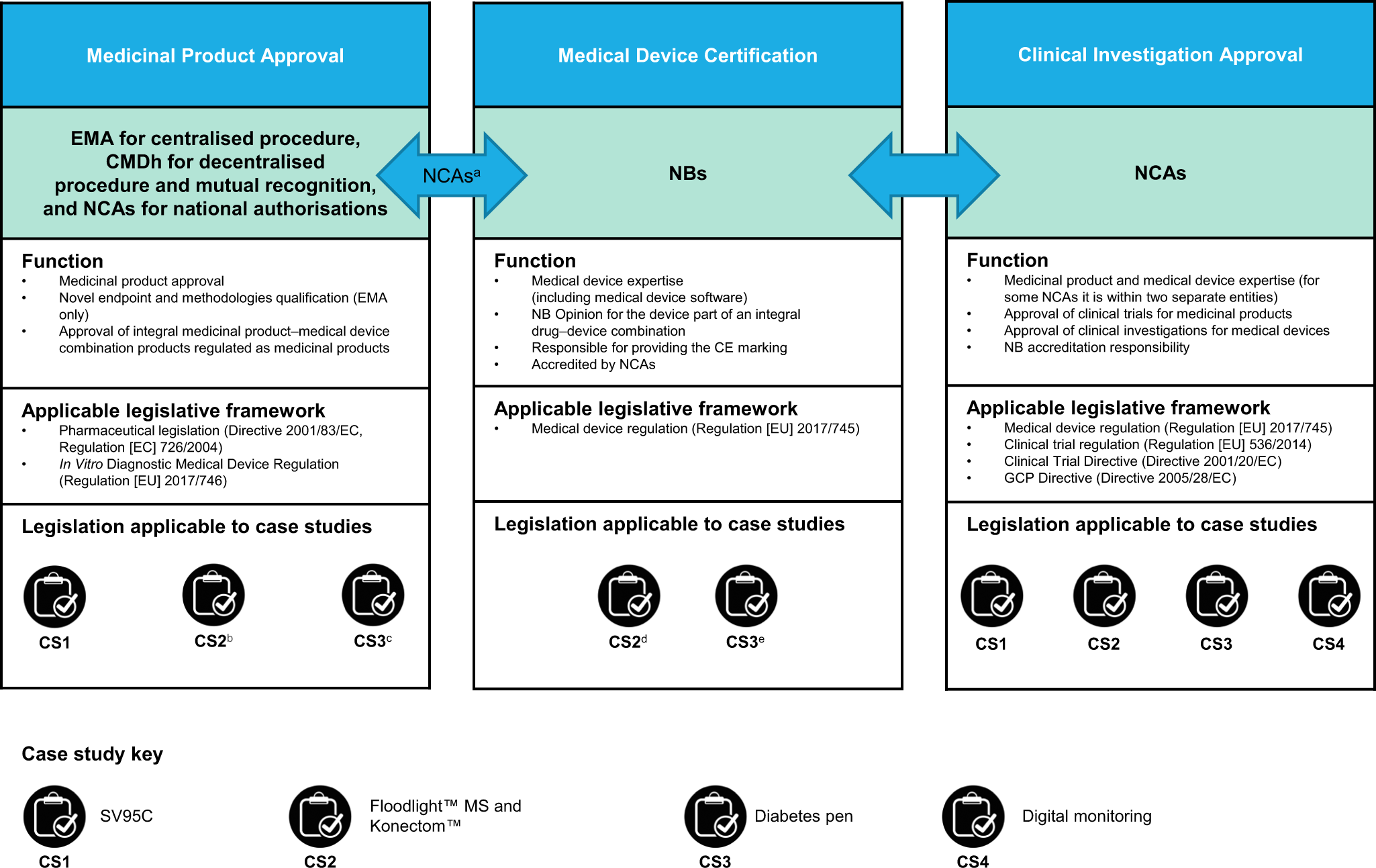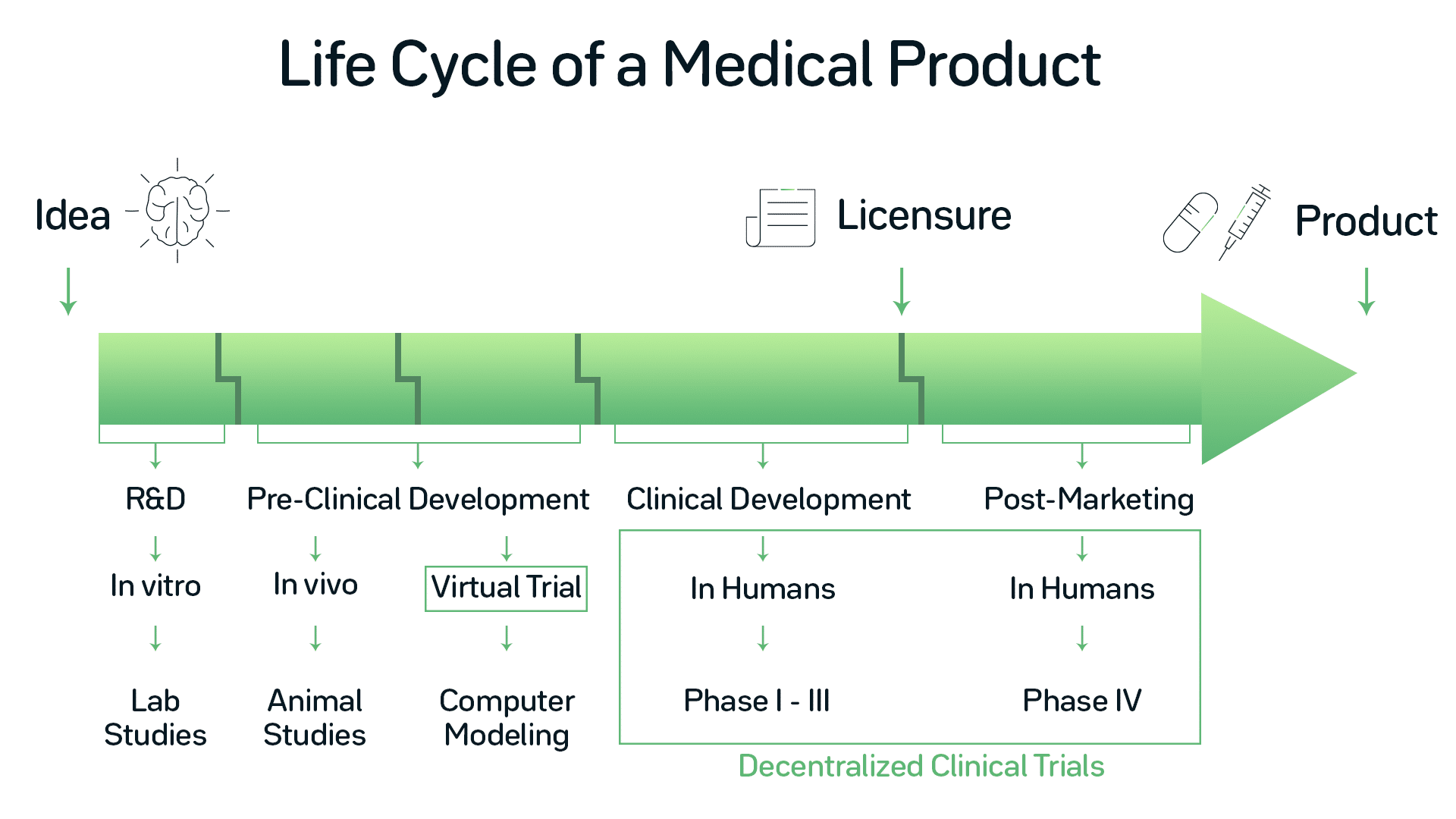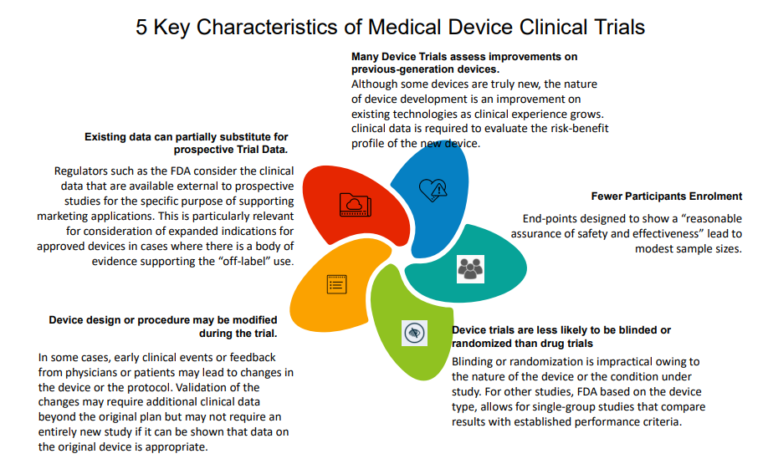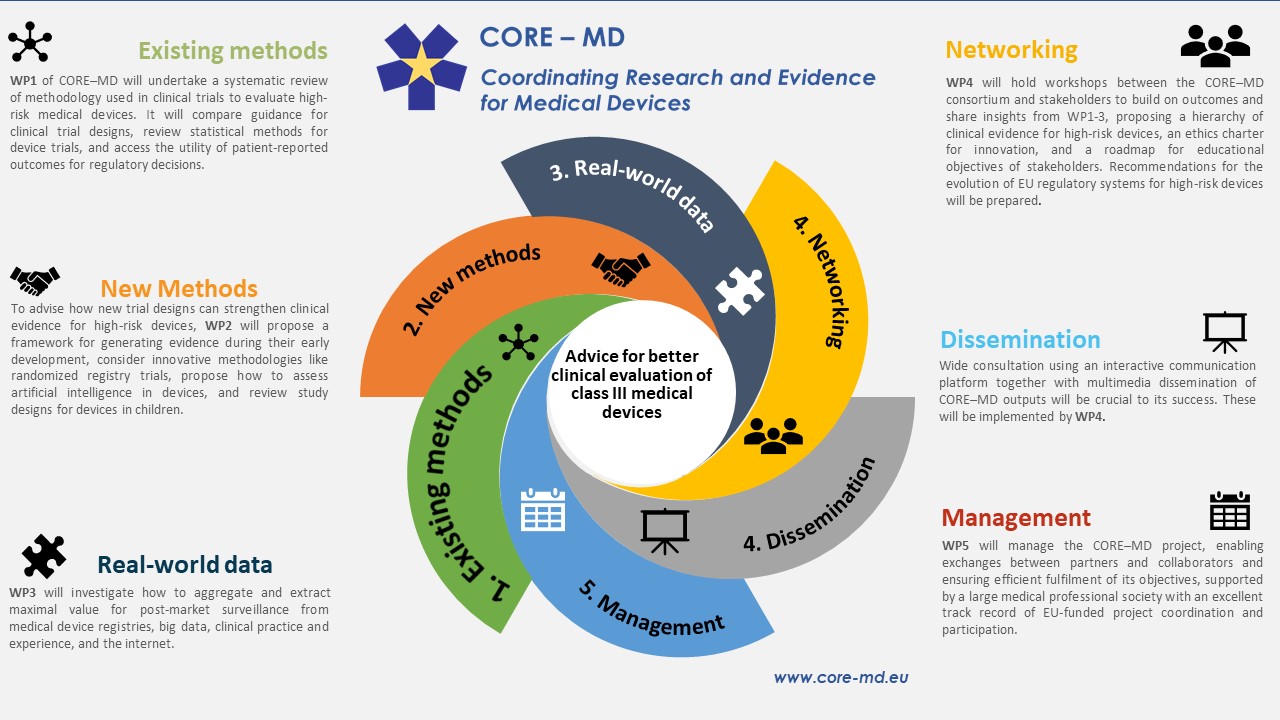
Registration, publication, and outcome reporting among pivotal clinical trials that supported FDA approval of high-risk cardiovascular devices before and after FDAAA | Trials | Full Text

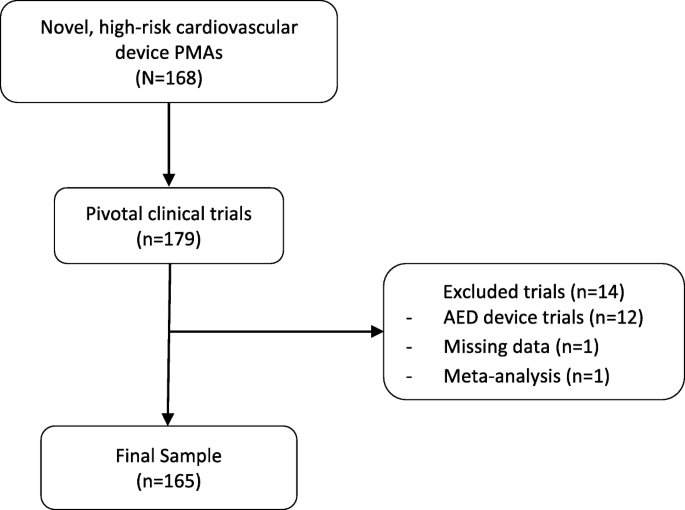
flowchart showing selection of clinical studies of new medical devices | Download Scientific Diagram
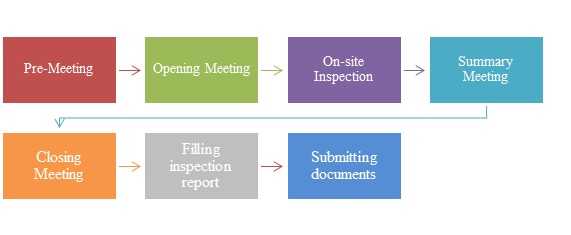
On-site Inspection Procedure of Medical Device Clinical Trials in China - Regulatory News - Medical Devices - CIRS Group

Regulation of Medical Devices and their Clinical Trials Studies in the USA: An Update | Bentham Science

Medical Device Development, Class I, II, III, 510(k), PMA, Significant Risk (SR), Nonsignificant Risk (NSR) – www.ClinicalResearchAssociateCRA.com – Insight into the world of clinical research from a CRA